The Importance of Molecular Imaging Characteristics for the Diagnosis and Management of Tumor-induced Osteomalacia
DOI:
https://doi.org/10.59667/sjoranm.v24i1.18Keywords:
tumor-induced osteomalacia, phosphaturic mesenchymal tumors, 68Ga-DOTATATE, positron emission tomography, computed tomographyAbstract
Objective: Tumor-induced osteomalacia (TIO) is a paraneoplastic syndrome associated with the overproduction of fibroblast growth factor 23 secondary to phosphaturic mesenchymal tumors (PMT). Our goal was to describe the morphometabolic characterization and histopathological correlation of images obtained from patients with suspected TIO using gallium-68 (68Ga) 1,4,7,10-tetraazacyclododecane-1,4,7,10-tetraacetic acid (DOTA)-octreotate(68Ga‑DOTATATE) positron emission tomography/computed tomography (PET/CT) in a referral center in Argentina.
Methods: A prospective, descriptive study with patients suspected of TIO who were referred to confirm the presence of primary lesions by 68Ga-DOTATATE PET/CT.
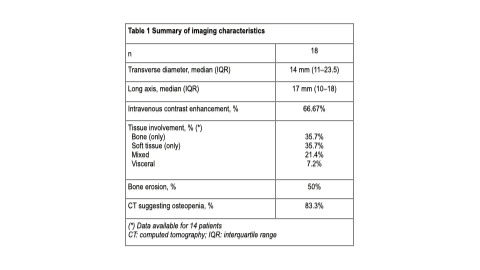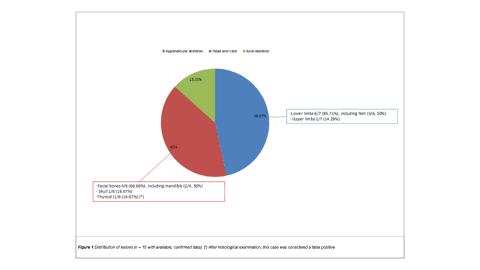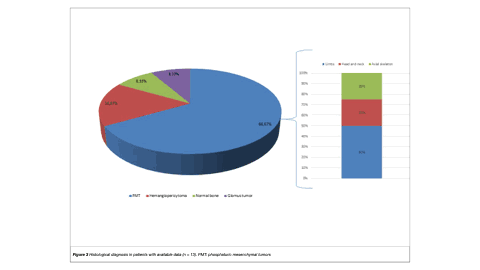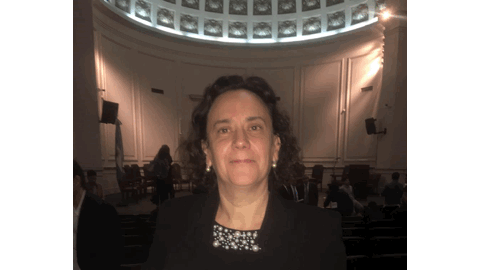
Results: Eighteen patients were included (female: 72.22%; median age: 47.5 years [range: 41.5–54]). The median maximum standardized uptake value (SUVmax) was 17.2 (interquartile range: 6.27–30.6). Lesions diagnosed by 68Ga-DOTATATE PET/CT were predominantly localized in the appendicular skeleton. Most patients had one identifiable lesion. Lesions were focal and well-limited in 66.67% of cases. Histopathological data were available for 13 patients. PMT was diagnosed in 61.54% of cases; in this subgroup, 25% had lesions showing ill-defined borders and confirmed bone erosion. A numerically, non-significant higher SUVmax was found in patients with PMT. Also, a trend towards isolated soft tissue involvement was more commonly observed among these patients.
Conclusion: In our patients with suspected TIO evaluated by 68Ga-DOTATATE PET/CT, a greater number of lesions were unique, well-defined, and localized in the appendicular skeleton. Nevertheless, ill-defined borders, including bone erosion, were reported in 25% of patients with confirmed PMTs. 68GaDOTATATE PET/CT is a valuable diagnostic tool for patients with suspected TIO. Further research is warranted.
References
1. Minisola S, Fukumoto S, Xia W, Corsi A, Colangelo L, Scillitani A, et al. Tumor-induced Osteomalacia: A Comprehensive Review. Endocr Rev. 2023;44(2):323-53, doi: https://doi.org/10.1210/endrev/bnac026
2. Florenzano P, Hartley IR, Jimenez M, Roszko K, Gafni RI, Collins MT Tumor-Induced Osteomalacia. Calcif Tissue Int. 2021;108(1):128-42, https://doi.org/10.1007/s00223-020-00691-6
3. Folpe AL, Fanburg-Smith JC, Billings SD, Bisceglia M, Bertoni F, Cho JY, et al. Most osteomalacia-associated mesenchymal tumors are a single histopathologic entity: an analysis of 32 cases and a comprehensive review of the literature. Am J Surg Pathol. 2004;28(1):1-30, https://doi.org/10.1097/00000478-200401000-00001
4. Shimada T, Mizutani S, Muto T, Yoneya T, Hino R, Takeda S, et al. Cloning and characterization of FGF23 as a causative factor of tumor-induced osteomalacia. Proc Natl Acad Sci U S A. 2001;98(11):6500-5, https://doi.org/10.1073/pnas.101545198
5. Chong J, Leung B, Poole P Phosphodiesterase 4 inhibitors for chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2013;(11):CD002309, https://doi.org/10.1002/14651858.CD002309.pub4
6. Clifton-Bligh RJ, Hofman MS, Duncan E, Sim I-W, Darnell D, Clarkson A, et al. Improving diagnosis of tumor-induced osteomalacia with Gallium-68 DOTATATE PET/CT. J Clin Endocrinol Metab. 2013;98(2):687-94, https://doi.org/10.1210/jc.2012-3642
7. Kobayashi H, Makise N, Ushiku T, Ito N, Koga M, Shinoda Y, et al. Infiltrative nature of tumor-induced osteomalacia lesions in bone: Correlation between radiological and histopathological features. Journal of Orthopaedic Science. 2019;24(5):900-5, https://doi.org/10.1016/j.jos.2019.02.005
8. Zuo Q, Wang H, Li W, Niu X, Huang Y, Chen J, et al. Treatment and outcomes of tumor-induced osteomalacia associated with phosphaturic mesenchymal tumors: retrospective review of 12 patients. BMC Musculoskelet Disord. 2017;18(1), https://doi.org/10.1186/s12891-017-1756-1
9. Jerkovich F, Nuñez S, Mocarbel Y, Pignatta A, Elías N, Cassinelli H, et al. Burden of Disease in Patients With Tumor‐Induced Osteomalacia. JBMR Plus. 2021;5(2):e10436, https://doi.org/10.1002/jbm4.10436
10. Zanchetta MB, Jerkovich F, Nuñez S, Mocarbel Y, Pignatta A, Elías N, et al. Impaired bone microarchitecture and strength in patients with tumor-induced osteomalacia. Journal of Bone and Mineral Research. 2020;36(8):1502-9, https://doi.org/10.1002/jbmr.4325
11. Hesse E, Rosenthal H, Bastian L Radiofrequency ablation of a tumor causing oncogenic osteomalacia. N Engl J Med. 2007;357(4):422-4, https://doi.org/10.1056/NEJMc070347
12. Gonzalez MR, Patel N, Connolly JJ, Hung YP, Chang CY, Lozano-Calderon SA Phosphaturic mesenchymal tumor: management and outcomes of ten patients treated at a single institution. Skeletal Radiol. 2024;53(8):1495-506, https://doi.org/10.1007/s00256-024-04614-6
13. Dahir K, Zanchetta MB, Stanciu I, Robinson C, Lee JY, Dhaliwal R, et al. Diagnosis and Management of Tumor-induced Osteomalacia: Perspectives From Clinical Experience. Journal of the Endocrine Society. 2021;5(9):bvab099, https://doi.org/10.1210/jendso/bvab099
14. Liu Y, He H, Zhang C, Zeng H, Tong X, Liu Q Phosphaturic Mesenchymal Tumors: Rethinking the Clinical Diagnosis and Surgical Treatment. JCM. 2022;12(1):252, https://doi.org/10.3390/jcm12010252
15. Abrahamsen B, Smith CD, Minisola S Epidemiology of Tumor-Induced Osteomalacia in Denmark. Calcif Tissue Int. 2021;109(2):147-56, https://doi.org/10.1007/s00223-021-00843-2
16. Nandam N, Ejaz S, Ahrens W, Styner M A Normal FGF23 Does Not Preclude Tumor‐Induced Osteomalacia. JBMR Plus. 2021;5(2), https://doi.org/10.1002/jbm4.10438
Downloads
Published
Data Availability Statement
The datasets generated during the current study are available from the corresponding author on reasonable request.
License
Copyright (c) 2025 Prof. Dr. María Bastianello, MD PhD, Roxana Chirico, Matías Cimín, María Belén Zanchetta

This work is licensed under a Creative Commons Attribution 4.0 International License.
This license requires that reusers give credit to the creator. It allows reusers to distribute, remix, adapt, and build upon the material in any medium or format, even for commercial purposes.








