The Radiologically Equivocal Bone Lesions Finally Diagnosed by Bone Scan
DOI:
https://doi.org/10.59667/sjoranm.v19i1.14Keywords:
bone scan, equivocal bone lesions, accurate diagnosisAbstract
Bone scintigraphy remains the second highest volume procedure in nuclear medicine laboratories with diverse applications. Bone scans are highly sensitive and can detect abnormalities much earlier than conventional X-rays. They provide a full-body image, allowing for the assessment of multiple bone sites simultaneously. they can also show specific patterns associated with specific diseases, eliminating ambiguity in diagnosis and establishing a specific diagnosis. Bone scan may be the final station to confirm the diagnosis of certain bone lesions that appear equivocal on other imaging modalities. There are some conditions where bone scans can be considered accurate and guide precise diagnosis, particularly when interpreted in conjunction with clinical findings and/or other imaging modalities as bone metastases, myositis ossificans, osteomyelitis, discitis, avascular necrosis, metabolic bone disease, fibrous dysplasia, osteopetrosis, stress fractures, Rheumatoid arthritis, reflex sympathetic dystrophy, transient migratory osteoporosis, hypertrophic osteoarthropathy, osteoid osteoma, condylar hyperplasia and osteopoikilosis.
References
1. Bridges, R. L., Wiley, C. R., Christian, J. C., & Strohm, A. P. (2007). An introduction to Na18F bone scintigraphy: basic principles, advanced ima-ging concepts, and case examples. Journal of Nuclear Medicine Technology, 35(2), 64–76. https://doi.org/10.2967/jnmt.106.032870
2. Cederquist, G. Y., & Escorcia, F. E. (2023). A brief history of radiopharmaceutical therapy. In Radiopharmaceutical Therapy (pp. 13–38). Book: ISBN 978-3-031-39004-3 https://link.springer.com/chapter/10.1007/978-3-031-39005-0_2
3. Drozdovitch, V., Brill, A. B., Callahan, R. J., Clanton, J. A., DePietro, A., Goldsmith, S. J., ... & Pon-to, J. A. (2015). Use of radiopharmaceuticals in diagnostic nuclear medicine in the United States: 1960–2010. Health Physics, 108(5), 520–537. https://doi.org/10.1097/HP.0000000000000261
4. Bouchareb, Y., AlSaadi, A., Zabah, J., Jain, A., Al-Jabri, A., Phiri, P., Shi, J. Q., Delanerolle, G., & Sirasanagandla, S. R. (2024). Technological advances in SPECT and SPECT/CT imaging. Diagnostics, 14(13), 1431. https://doi.org/10.3390/diagnostics14131431
5. Fogelman, I., & Carr, D. (1980). A comparison of bone scanning and radiology in the evaluation of patients with metabolic bone disease. Clinical Radiology, 31(3), 321–326. https://doi.org/10.1016/S0009-9260(80)80230-3 https://www.clinicalradiologyonline.net/article/S0009-9260(80)80230-3/pdf
6. Brenner, A. I., Koshy, J., Morey, J., Lin, C., & DiPoce, J. (2012). The bone scan. In Seminars in Nuclear Medicine (Vol. 42, No. 1, pp. 11–26). WB Saunders. https://doi.org/10.1053/j.semnuclmed.2011.07.005
7. Buijs, J. T., & van der Pluijm, G. (2009). Osteotropic cancers: from primary tumor to bone. Cancer Letters, 273(2), 177–193. https://doi.org/10.1016/j.canlet.2008.05.044
8. Fidler, I. J. (2003). The pathogenesis of cancer metastasis: the 'seed and soil' hypothesis revisited. Nature Reviews Cancer, 3(6), 453–458.; https://doi.org/10.1038/nrc1098
9. Coleman, R. E., Brown, J., & Holen, I. (2020). Bone metastases. In Abeloff's Clinical Oncology (pp. 809–830). https://doi.org/10.1016/B978-0-323-47674-4.00056-6
10. Selvaggi, G., & Scagliotti, G. V. (2005). Management of bone metastases in cancer: a review. Critical Reviews in Oncology/Hematology, 56(3), 365–378. https://doi.org/10.1016/j.critrevonc.2005.03.011
11. Askari, E., Shakeri, S., Roustaei, H., Fotouhi, M., Sadeghi, R., Harsini, S., & Vali, R. (2024). Superscan pattern on bone scintigraphy: A comprehensive review. Diagnostics, 14(19), 2229. https://doi.org/10.3390/diagnostics14192229
12. Khanna, G. (2010). Imaging of pediatric bone tumors: Osteosarcoma and Ewing sarcoma. In Evidence-Based Imaging in Pediatrics: Optimi-zing Imaging in Pediatric Patient Care (pp. 259–273). Springer. https://link.springer.com/chapter/10.1007/978-1-4419-0922-0_18
13. Chua, S., Gnanasegaran, G., & Cook, G. J. R. (2009). Miscellaneous cancers (lung, thyroid, renal cancer, myeloma, and neuroendocrine tumors): Role of SPECT and PET in imaging bone me-tastases. Seminars in Nuclear Medicine, 39(6), 416–430. https://doi.org/10.1053/j.semnuclmed.2009.08.003
14. Mehta, K., Haller, J. O., & Legasto, A. C. (2003). Imaging neuroblastoma in children. Critical Reviews in Computed Tomography, 44(1), 47–61. https://pubmed.ncbi.nlm.nih.gov/12627783/
15. González, C. S., Vivero, C. M., & Castro, J. L. (2022). Paraneoplastic syndromes review: The great forgotten ones. Critical Reviews in Onco-logy/Hematology, 174, 103676. https://doi.org/10.1016/j.critrevonc.2022.103676
16. Ma, G. M., Chow, J. S., & Taylor, G. A. (2019). Review of paraneoplastic syndromes in chil-dren. Pediatric Radiology, 49(4), 534–550. https://doi.org/10.1007/s00247-019-04371-y
17. Khalatbari Kani, K., Porrino, J. A., Mulligan, M. E., & Chew, F. S. (2023). Paraneoplastic musculo-skeletal disorders: Review and update for radiologists. Skeletal Radiology, 52(3), 421–433. https://doi.org/10.1007/s00256-022-04074-w
18. Morton, K. A. (1999). Extra-skeletal uptake of bone agents. Journal of Nuclear Medicine Techno-logy, 27(1), 51–53. https://tech.snmjournals.org/content/27/1/51
19. Didona, D., Fania, L., Didona, B., Eming, R., Hertl, M., & Di Zenzo, G. (2020). Paraneoplastic dermatoses: A brief general review and an extensive analysis of paraneoplastic pemphi-gus and paraneoplastic dermatomyositis. International Journal of Molecular Sciences, 21(6), 2178. https://doi.org/10.3390/ijms21062178
20. Chong, W. H., Molinolo, A. A., Chen, C. C., & Collins, M. T. (2011). Tumor-induced osteoma-lacia. Endocrine-Related Cancer, 18(3), R53–R77. https://doi.org/10.1530/ERC-11-0006
21. Walczak, B. E., Johnson, C. N., & Howe, B. M. (2015). Myositis ossificans. JAAOS - Journal of the American Academy of Orthopaedic Sur-geons, 23(10), 612–622. https://doi.org/10.5435/JAAOS-D-14-00269
22. Shirkhoda, A., Armin, A. R., Bis, K. G., Makris, J., Irwin, R. B., & Shetty, A. N. (1995). MR imaging of myositis ossificans: Variable patterns at different stages. Journal of Magnetic Reso-nance Imaging, 5(3), 287–292. https://doi.org/10.1002/jmri.1880050312
23. Hanisch, M., Hanisch, L., Fröhlich, L. F., Werk-meister, R., Bohner, L., & Kleinheinz, J. (2018). Myositis ossificans traumatica of the mastica-tory muscles: Etiology, diagnosis and treat-ment. Head & Face Medicine, 14, Article 23. https://doi.org/10.1186/s13005-018-0180-6
24. Saad, A., Azzopardi, C., Patel, A., Davies, A. M., & Botchu, R. (2021). Myositis ossificans revisited – The largest reported case series. Journal of Clinical Orthopaedics and Trauma, 17, 123–127. https://doi.org/10.1016/j.jcot.2021.03.005
25. Thomas, E. A., Cassar-Pullicino, V. N., & McCall, I. W. (1991). The role of ultrasound in the early diagnosis and management of heterotopic bone formation. Clinical Radiology, 43(3), 190–196. https://doi.org/10.1016/S0009-9260(05)80478-7
26. Lew, D. P., & Waldvogel, F. A. (2004). Osteomyelitis. The Lancet, 364(9431), 369–379. https://doi.org/10.1016/S0140-6736(04)16727-5
27. Maffulli, N., Papalia, R., Zampogna, B., Torre, G., Albo, E., & Denaro, V. (2016). The management of osteomyelitis in the adult. The Surgeon, 14(6), 345–360. https://doi.org/10.1016/j.surge.2015.12.005
28. Smith, B. J., Buchanan, G. S., & Shuler, F. D. (2016). A comparison of imaging modalities for the diagnosis of osteomyelitis. Marshall Journal of Medicine, 2(3), Article 10. https://doi.org/10.18590/mjm.2016.vol2.iss3.10
29. Harmer, J. L., Pickard, J., & Stinchcombe, S. J. (2011). The role of diagnostic imaging in the evaluation of suspected osteomyelitis in the foot: A critical review. The Foot, 21(3), 149–153. https://doi.org/10.1016/j.foot.2010.11.005
30. Wheat, J. (1985). Diagnostic strategies in osteo-myelitis. The American Journal of Medicine, 78 (6), 218–224. https://doi.org/10.1016/0002-9343(85)90388-2
31. Schauwecker DS, Braunstein EM, Wheat LJ. Dia-gnostic imaging of osteomyelitis. Infectious Disease Clinics of North America. 1990 Sep 1;4(3):441-63. https://www.sciencedirect.com/science/article/pii/S0891552020303561
32. Sammak, B., Abd El Bagi, M. A., Al Shahed, M., Hamilton, D., Al Nabulsi, J., Youssef, B., & Al Thagafi, M. (1999). Osteomyelitis: A review of currently used imaging techniques. European Radiology, 9(5), 894–900. https://doi.org/10.1007/s003300050763
33. Dinh, M. T., Abad, C. L., & Safdar, N. (2008). Diagnostic accuracy of the physical examin-ation and imaging tests for osteomyelitis underlying diabetic foot ulcers: meta-analysis. Clinical Infectious Diseases, 47(4), 519–527. https://doi.org/10.1086/590011
34. García-Arias, M., Balsa, A., & Mola, E. M. (2011). Septic arthritis. Best Practice & Research Clinical Rheumatology, 25(3), 407–421. https://doi.org/10.1016/j.berh.2011.02.001
35. Fatima, F., Fei, Y., Ali, A., Mohammad, M., Erlands-son, M. C., Bokarewa, M. I., Nawaz, M., Valadi, H., Na, M., & Jin, T. (2017). Radiological fea-tures of experimental staphylococcal septic ar-thritis by micro computed tomography scan. PLOS ONE, 12(2), e0171222. https://doi.org/10.1371/journal.pone.0171222
36. Lam, K. S., & Webb, J. K. (2004). Discitis. Hospital Medicine, 65(5), 280–286. https://doi.org/10.12968/hosp.2004.65.5.13703
37. Hinners J. Vertebral osteomyelitis and the role of imaging. Radiologic Technology. 2017 May 1;88(5):519-35. https://pubmed.ncbi.nlm.nih.gov/28500093/
38. Elgazzar, A. H. (2024). Diagnosis of inflammatory bone diseases. In Orthopedic Nuclear Medi-cine (3. Aufl., S. 35–118). Springer. https://doi.org/10.1007/978-3-031-51932-1_2
39. Tali, E. T. (2004). Spinal infections. European Journal of Radiology, 50(2), 120–133. https://doi.org/10.1016/j.ejrad.2003.10.022
40. Herman, K., Pękala, P., Szwedowski, D., Grabowski, R., & Cholewiński, J. (2022). Avascular necro-sis. In Joint Function Preservation: A Focus on the Osteochondral Unit (S. 161–171). Springer. A Focus on the Osteochondral Unit
41. Wells, M. E., & Dunn, J. C. (2022). Pathophysiology of avascular necrosis. Hand Clinics, 38(4), 367–376. https://doi.org/10.1016/j.hcl.2022.03.011
42. Lohiya, A., Dhaniwala, N., Dudhekar, U., Goyal, S., & Patel, S. K. (2023). A comprehensive review of treatment strategies for early avascular necro-sis. Cureus, 15(12), e50510. https://doi.org/10.7759/cureus.50510
43. Bedair, E. M., Almaslamani, N. J., & Yassin, M. (2023). Radiological manifestation of avascular necrosis (AVN) in sickle cell disease (SCD): A review of diagnostic imaging. Acta Bio Medica: Atenei Parmensis, 94(3), e2023177. https://doi.org/10.23750/abm.v94i3.14714
44. Imhof, H., Breitenseher, M., Trattnig, S., Kramer, J., Hofmann, S., Plenk, H., Schneider, W., & Engel, A. (1997). Imaging of avascular necrosis of bone. European Radiology, 7(2), 180–186. https://doi.org/10.1007/s003300050131
45. Collier, B. D., Carrera, G. F., Johnson, R. P., Isitman, A. T., Hellman, R. S., Knobel, J., Finger, W. A., Gonyo, J. E., & Malloy, P. J. (1985). Detection of femoral head avascular necrosis in adults by SPECT. Journal of Nuclear Medicine, 26(9), 979–987. https://jnm.snmjournals.org/content/jnumed/26/9/979.full.pdf
46. Ryu, J. S., Kim, J. S., Moon, D. H., Kim, S. M., Shin, M. J., Chang, J. S., ... & Lee, H. K. (2002). Bone SPECT is more sensitive than MRI in the detection of early osteonecrosis of the femoral head after renal transplantation. Journal of Nuclear Medicine, 43(8), 1006–1011 https://pubmed.ncbi.nlm.nih.gov/12163624/
47. Conklin, J. J., Alderson, P. O., Zizic, T. M., Hunger-ford, D. S., Densereaux, J. Y., Gober, A., & Wagner, H. N. (1983). Comparison of bone scan and radiograph sensitivity in the de-tection of steroid-induced ischemic necrosis of bone. Radiology, 147(1), 221–226. https://doi.org/10.1148/radiology.147.1.6219425
48. Beltran, J., Herman, L. J., Burk, J. M., Zuelzer, W. A., Clark, R. N., Lucas, J. G., Weiss, L. D., & Yang, A. (1988). Femoral head avascular necrosis: MR imaging with clinical-pathologic and radio-nuclide correlation. Radiology, 166 (1), 215–220. https://doi.org/10.1148/radiology.166.1.3336682
49. Sarikaya, I., Sarikaya, A., & Holder, L. E. (2001). The role of single photon emission computed to-mography in bone imaging. Seminars in Nu-clear Medicine, 31(1), 3–16. https://doi.org/10.1053/snuc.2001.18736
50. Imel EA, DiMeglio LA, Burr DB. Metabolic bone diseases. InBasic and applied bone biology 2014 Jan 1 (pp. 317-344). Academic Press https://doi.org/10.1016/B978-0-12-416015-6.00016-2
51. Gopinath, P., & Mihai, R. (2011). Hyperpara-thyroidism. Surgery (Oxford), 29(9), 451–458. https://doi.org/10.1016/j.mpsur.2011.06.015
52. Adams, J. E. (2011). Radiology of rickets and osteo-malacia. In D. Feldman, J. W. Pike, & M. Hewi-son (Eds.), Vitamin D (3rd ed., pp. 861–889). Academic Press. https://doi.org/10.1016/B978-0-12-381978-9.10049-6
53. Rachner, T. D., Khosla, S., & Hofbauer, L. C. (2011). Osteoporosis: Now and the future. The Lancet, 377(9773), 1276–1287. https://doi.org/10.1016/S0140-6736(10)62349-5
54. Ralston, S. H. (2013). Clinical practice. Paget's di-sease of bone. New England Journal of Medi-cine, 368(7), 644–650. https://doi.org/10.1056/NEJMcp1204713
55. Elder, G. (2002). Pathophysiology and recent advan-ces in the management of renal osteodystro-phy. Journal of Bone and Mineral Research, 17(12), 2094–2105. https://doi.org/10.1359/jbmr.2002.17.12.2094
56. Rauch, F., & Glorieux, F. H. (2004). Osteogenesis imperfecta. The Lancet, 363(9418), 1377–1385. https://doi.org/10.1016/S0140-6736(04)16051-0
57. Mornet, E. (2008). Hypophosphatasia. Best Practice & Research Clinical Rheumatology, 22(1), 113–127. https://doi.org/10.1016/j.berh.2007.11.003
58. Patel, A. A., Ramanathan, R., Kuban, J., & Willis, M. H. (2015). Imaging findings and evaluation of metabolic bone disease. Advances in Radio-logy, 2015, 812794. https://doi.org/10.1155/2015/812794
59. Fogelman, I. (1987). The bone scan in metabolic bone disease. In Bone Scanning in Clinical Practice (pp. 73–87). Springer London. https://doi.org/10.1007/978-1-4471-1407-9_7
60. Misiorowski, W., Czajka-Oraniec, I., Kochman, M., Zgliczyński, W., & Bilezikian, J. P. (2017). Osteitis fibrosa cystica—a forgotten radiologi-cal feature of primary hyperparathyroidism. Endocrine, 58(2), 380–385. https://doi.org/10.1007/s12020-017-1414-2
61. Silverberg, S. J., & Bilezikian, J. P. (1996). Evaluation and management of primary hyperpara-thyroidism. The Journal of Clinical Endocrino-logy & Metabolism, 81(6), 2036–2040. https://doi.org/10.1210/jcem.81.6.8964825
62. Jennings, E., Buckberry, J., & Brickley, M. B. (2018). Radiographically recognizable? An investiga-tion into the appearance of osteomalacic pseudofractures. International Journal of Paleopathology, 23, 26–31. https://doi.org/10.1016/j.ijpp.2017.12.003
63. Yang, M., Doshi, K. B., Roarke, M. C., & Nguyen, B. D. (2019). Molecular imaging in diagnosis of tumor-induced osteomalacia. Current Pro-blems in Diagnostic Radiology, 48(4), 379–386. https://doi.org/10.1067/j.cpradiol.2018.06.005
64. Adams, J. E. (2013). Advances in bone imaging for osteoporosis. Nature Reviews Endocrinology, 9(1), 28–42. https://doi.org/10.1038/nrendo.2012.217
65. Krestan, C., & Hojreh, A. (2009). Imaging of insufficiency fractures. European Journal of Radiology, 71(3), 398–405. https://doi.org/10.1016/j.ejrad.2008.04.059
66. Lombardi, A. F., Aihara, A. Y., Fernandes, A. D., & Cardoso, F. N. (2022). Imaging of Paget’s di-sease of bone. Radiologic Clinics of North America, 60(4), 561–573. https://doi.org/10.1016/j.rcl.2022.02.005
67. Mirra, J. M., Brien, E. W., & Tehranzadeh, J. (1995). Paget's disease of bone: Review with em-phasis on radiologic features, Part II. Skeletal Radiology, 24, 173–184. https://doi.org/10.1007/BF00228919
68. Riddle, N. D., & Bui, M. M. (2013). Fibrous dys-plasia. Archives of Pathology & Laboratory Medicine, 137(1), 134–138. https://doi.org/10.5858/arpa.2012.0013-RS
69. Lee, S. E., Lee, E. H., Park, H., Sung, J. Y., Lee, H. W., Kang, S. Y., ... & Ahn, G. (2012). The dia-gnostic utility of the GNAS mutation in patients with fibrous dysplasia: Meta-analysis of 168 sporadic cases. Human Pathology, 43(8), 1234–1242. https://doi.org/10.1016/j.humpath.2011.09.012
70. Parekh, S. G., Donthineni-Rao, R., Ricchetti, E., & Lackman, R. D. (2004). Fibrous dysplasia. Journal of the American Academy of Ortho-paedic Surgeons, 12(5), 305–313. https://doi.org/10.5435/00124635-200409000-00005
71. Lisle, D. A., Monsour, P. A., & Maskiell, C. D. (2008). Imaging of craniofacial fibrous dysplasia. Jour-nal of Medical Imaging and Radiation Onco-logy, 52(4), 325–332. https://doi.org/10.1111/j.1440-1673.2008.01963.x
72. Zhibin, Y., Quanyong, L., Libo, C., Jun, Z., Hankui, L., Jifang, Z., & Ruisen, Z. (2004). The role of radionuclide bone scintigraphy in fibrous dysplasia of bone. Clinical Nuclear Medicine, 29(3), 177–180. https://doi.org/10.1097/01.rlu.0000113856.77103.7e
73. Arumugam, E., Harinathbabu, M., Thillaigovindan, R., & Prabhu, G. (2015). Marble bone disease: A rare bone disorder. Cureus, 7(10), e339. https://doi.org/10.7759/cureus.339
74. Whyte, M. P. (2023). Osteopetrosis: Discovery and early history of “marble bone disease”. Bone, 171, 116737. https://doi.org/10.1016/j.bone.2023.116737
75. Sit, C., Agrawal, K., Fogelman, I., & Gnanasegaran, G. (2015). Osteopetrosis: Radiological & radio-nuclide imaging. Indian Journal of Nuclear Me-dicine, 30(1), 55–58. https://doi.org/10.4103/0972-3919.147544
76. Patel, D. S., Roth, M., & Kapil, N. (2011). Stress fractures: Diagnosis, treatment, and preven-tion. American Family Physician, 83(1), 39–46. https://pubmed.ncbi.nlm.nih.gov/21888126/
77. Anderson, M. W., & Greenspan, A. (1996). Stress fractures. Radiology, 199(1), 1–12. https://doi.org/10.1148/radiology.199.1.8633129
78. Moran, D. S., Evans, R. K., & Hadad, E. (2008). Ima-ging of lower extremity stress fracture injuries. Sports Medicine, 38(4), 345–356. https://doi.org/10.2165/00007256-200838040-00005
79. Wright, A. A., Hegedus, E. J., Lenchik, L., Kuhn, K. J., Santiago, L., & Smoliga, J. M. (2016). Dia-gnostic accuracy of various imaging modalities for suspected lower extremity stress fractures: A systematic review with evidence-based re-commendations for clinical practice. The Ame-rican Journal of Sports Medicine, 44(1), 255–263. https://doi.org/10.1177/0363546515574066
80. Prather, J. L., Nusynowitz, M. L., Snowdy, H. A., Hughes, A. D., McCartney, W. H., & Bagg, R. J. (1977). Scintigraphic findings in stress frac-tures. JBJS, 59(7), 869–874. https://pubmed.ncbi.nlm.nih.gov/908718/
81. Radu, A. F., & Bungau, S. G. (2021). Management of rheumatoid arthritis: An overview. Cells, 10(11), 2857. https://doi.org/10.3390/cells10112857
82. Rosenthall, L. (1987). The bone scan in arthritis. In Bone Scanning in Clinical Practice (pp. 133–150). Springer. https://doi.org/10.1007/978-1-4471-1407-9_11
83. Pickhardt, P. J., & Shapiro, B. (1996). Three-phase skeletal scintigraphy in gouty arthritis: An example of potential diagnostic pitfalls in radiopharmaceutical imaging of the extremities for infection. Clinical Nuclear Medicine, 21(1), 33–39. https://doi.org/10.1097/00003072-199601000-00009
84. Marshall, A. T., & Crisp, A. J. (2000). Reflex sympa-thetic dystrophy. Rheumatology, 39(7), 692–695. https://doi.org/10.1093/rheumatology/39.7.692
85. Michael, d'A. S. H. (2019). CRPS: What’s in a name? Taxonomy, epidemiology, neurologic, immune and autoimmune considerations. Regio-nal Anesthesia & Pain Medicine, 44(3), 376–387. https://doi.org/10.1136/rapm-2018-100064
86. Pachowicz, M., Nocuń, A., Postępski, J., Olesińska, E., Emeryk, A., & Chrapko, B. (2014). Complex Regional Pain Syndrome type I with atypical scintigraphic pattern—diagnosis and evalua-tion of the entity with three-phase bone scintigraphy: A case report. Nuclear Medicine Review, 17(2), 115–119. https://doi.org/10.5603/NMR.2014.0029
87. AlSharif, A., Akel, A. Y., Sheikh-Ali, R. F., Juweid, M. E., Hawamdeh, Z. M., Ajlouni, J. M., & ElHadidy, S. T. (2012). Is there a correlation between symptoms and bone scintigraphic findings in patients with complex regional pain syndrome? Annals of Nuclear Medicine, 26(8), 665–669. https://doi.org/10.1007/s12149-012-0623-2
88. Intenzo, C. M., Kim, S. M., & Capuzzi, D. M. (2005). The role of nuclear medicine in the evaluation of complex regional pain syndrome type I. Clinical Nuclear Medicine, 30(6), 400–407. https://doi.org/10.1097/01.rlu.0000162605.14734.11
89. Kaur, H., Muhleman, M. A., & Balon, H. R. (2017). Complex regional pain syndrome diagnosed with triple-phase bone scanning. Journal of Nuclear Medicine Technology, 45(3), 243–244. https://doi.org/10.2967/jnmt.117.192443
90. Tangella, A. V. (2023). Imaging modalities and their findings in patients with complex regional pain syndrome: A review. Cureus, 15(7), e42123. https://doi.org/10.7759/cureus.41747
91. Korompilias, A. V., Karantanas, A. H., Lykissas, M. G., & Beris, A. E. (2008). Transient osteopo-rosis. JAAOS Journal of the American Acade-my of Orthopaedic Surgeons, 16(8), 480–489. https://doi.org/10.5435/00124635-200808000-00007
92. Trevisan, C., Ortolani, S., Monteleone, M., & Marinoni, E. C. (2002). Regional migratory os-teoporosis: A pathogenetic hypothesis based on three cases and a review of the literature. Clinical Rheumatology, 21, 418–425. https://doi.org/10.1007/s100670200112
93. Martínez-Lavín, M. (2020). Hypertrophic osteoar-thropathy. Best Practice & Research Clinical Rheumatology, 34(3), 101507. https://doi.org/10.1016/j.berh.2020.101507
94. Rodríguez, N. G., Ruán, J. I., & Pérez, M. G. (2009). Primary hypertrophic osteoarthropathy (pachy-dermoperiostosis). Report of 2 familial cases and literature review. Reumatología Clínica (English Edition), 5(6), 259–263. https://doi.org/10.1016/S2173-5743(09)70134-0
95. Nahar, I., Al-Shemmeri, M., & Hussain, M. (2007). Secondary hypertrophic osteoarthropathy: New insights on pathogenesis and manage-ment. Gulf Journal of Oncology, 1, 71–76. https://pubmed.ncbi.nlm.nih.gov/20084716/
96. Yap, F. Y., Skalski, M. R., Patel, D. B., Schein, A. J., White, E. A., Tomasian, A., Masih, S., & Mat-cuk, G. R. Jr. (2017). Hypertrophic osteo-arthropathy: Clinical and imaging features. Ra-diographics, 37(1), 157–195. https://doi.org/10.1148/rg.2017160052
97. Savioli, S., French, C. N., Walker, E. A., Iloanusi Lo-gie, C., & Murphey, M. D. (2023). Hypertrophic osteoarthropathy. In Musculo-skeletal Imaging (pp. 1–15). Springer. https://doi.org/10.1007/978-3-030-57376-8_80-1
98. Hansen-Flaschen, J., & Nordberg, J. (1987). Club-bing and hypertrophic osteoarthropathy. Cli-nics in Chest Medicine, 8(2), 287–298. https://www.sciencedirect.com/science/article/abs/pii/S0272523121010236
99. Tepelenis, K., Skandalakis, G. P., Papathanakos, G., Kefala, M. A., Kitsouli, A., Barbouti, A., ... & Kit-soulis, P. (2021). Osteoid osteoma: An updated review of epidemiology, pathogenesis, clinical presentation, radiological features, and treat-ment options. In Vivo, 35(4), 1929–1938. https://doi.org/10.21873/invivo.12459
100. Lee, E. H., Shafi, M., & Hui, J. H. (2006). Osteoid osteoma: A current review. Journal of Pediatric Orthopaedics, 26(5), 695–700. https://doi.org/10.1097/01.bpo.0000233807.80046.7c
101. Boscainos, P. J., Cousins, G. R., Kulshreshtha, R., Oliver, T. B., & Papagelopoulos, P. J. (2013). Osteoid osteoma. Orthopedics, 36 (10), 792 –800. https://doi.org/10.3928/01477447-20130920-10
102. Smith, F. W., & Gilday, D. L. (1980). Scintigraphic appearances of osteoid osteoma. Radiology, 137(1), 191–195. https://doi.org/10.1148/radiology.137.1.6448428
103. Erba, P. A., Sollini, M., Zanca, R., Boni, R., Flynt, L., Lazzeri, E., Mariani, G., & Kuwert, T. (2019). Hybrid imaging and radionuclide therapy of musculoskeletal diseases. In G. Mariani, H. W. Strauss, P. A. Erba, & D. Volterrani (Eds.), Nuclear Medicine Textbook: Methodology and Clinical Applications (pp. 571–644). Springer. https://link.springer.com/chapter/10.1007/978-3-319-95564-3_24
104. Noordin, S., Allana, S., Hilal, K., Nadeem, N., Lak-dawala, R., Sadruddin, A., & Uddin, N. (2018). Osteoid osteoma: Contemporary manage-ment. Orthopedic Reviews, 10(3), 7496. https://doi.org/10.4081/or.2018.7496
105. Higginson, J. A., Bartram, A. C., Banks, R. J., & Keith, D. J. W. (2018). Condylar hyperplasia: Current thinking. British Journal of Oral and Maxillofacial Surgery, 56(8), 655–662. https://doi.org/10.1016/j.bjoms.2018.07.017
106. Karssemakers, L. H., Besseling, L. M., Schoon-made, L. J., Su, N., Nolte, J. W., Raijmakers, P. G., & Becking, A. G. (2024). Diagnostic accuracy of bone SPECT and SPECT/CT ima-ging in the diagnosis of unilateral condylar hyperplasia: A systematic review and meta-analysis. Journal of Cranio-Maxillofacial Sur-gery. https://doi.org/10.1016/j.jcms.2024.01.013
107. Maniskas, S. A., Ly, C. L., Pourtaheri, N., Parsaei, Y., & Steinbacher, D. M. (2020). Concurrent high condylectomy and orthognathic surgery for treatment of patients with unilateral condylar hyperplasia. Journal of Craniofacial Surgery, 31(8), 2217–2221. https://doi.org/10.1097/SCS.0000000000006987
108. Benli, I. T., Akalin, S., Boysan, E., Mumcu, E. F., Kis, M., & Turkoglu, D. (1992). Epidemiological, clinical and radiological aspects of osteopoi-kilosis. The Journal of Bone & Joint Surgery - British Volume, 74(4), 504–506. https://pubmed.ncbi.nlm.nih.gov/1624505/
109. Ellanti, P., Clarke, B., & Gray, J. (2010). Osteo-poikilosis. Irish Journal of Medical Science, 179,615–616. https://doi.org/10.1007/s11845-010-0595-y
110. Hill CE, McKee L. Osteopoikilosis: an important incidental finding. Injury. 2015 Jul 1;46(7): 1403-5. https://doi.org/10.1016/j.injury.2015.02.005
111. Carpintero P, Abad JA, Serrano P, Serrano JA, Rodríguez P, Castro L. Clinical features of ten cases of osteopoikilosis. Clinical rheumatology. 2004 Dec;23:505-8. https://doi.org/10.1007/s10067-004-0935-2
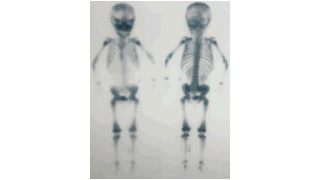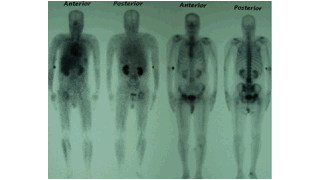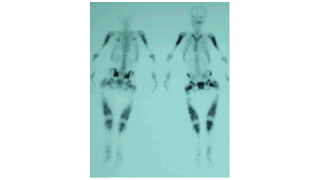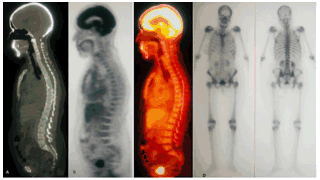
Downloads
Published
Issue
Section
License
Copyright (c) 2025 Mai Amr Elahmadawy, Mahmoud Akram, Ahmed Zaher

This work is licensed under a Creative Commons Attribution 4.0 International License.
This license requires that reusers give credit to the creator. It allows reusers to distribute, remix, adapt, and build upon the material in any medium or format, even for commercial purposes.








