The Impact of Diabetes and Diabetic Kidney Disease on Bone Mineral Density at the Lumbar Spine and Femoral Neck
DOI:
https://doi.org/10.59667/sjoranm.v27i1.26Keywords:
Osteoporosis, Diabetes, Chronic kidney disease, Osteopenia, Bone mineral density, DEXAAbstract
Background:
Type 2 diabetes mellitus (T2DM) and diabetic kidney disease are associated with metabolic disturbances that negatively affect bone health, increasing the risk of reduced bone mineral density (BMD), osteopenia, and osteoporosis. This study aimed to determine and compare the prevalence of osteopenia and osteoporosis among patients with T2DM with and without chronic kidney disease (CKD) and healthy non-diabetic controls.
Materials and Methods:
A total of 210 participants were enrolled and categorized into three groups: patients with T2DM and CKD, patients with T2DM without CKD, and healthy controls. BMD was measured at the lumbar spine and femoral neck using dual-energy X-ray absorptiometry (DEXA). Bone status was classified according to World Health Organization criteria: normal (T-score ≥ −1), osteopenia (T-score between −1 and −2.5), and osteoporosis (T-score < −2.5).
Results:
The prevalence of osteopenia and osteoporosis was significantly higher among diabetic patients compared to controls, with the highest rates observed in patients with diabetic kidney disease. Vertebral osteoporosis and osteopenia were present in 54% and 38% of patients with diabetic kidney disease, respectively. In patients with T2DM without CKD, osteoporosis and osteopenia were observed in 24% and 52% of cases, respectively. At the right femoral neck, osteoporosis prevalence was 24% in patients with diabetic kidney disease, 8% in patients with T2DM without CKD, and 4% in controls. Corresponding osteopenia rates were 38%, 42%, and 20%, respectively.
Conclusion:
Patients with T2DM, particularly those with concomitant CKD, exhibit a substantially higher prevalence of osteopenia and osteoporosis compared to non-diabetic individuals. These findings emphasize the need for routine bone health assessment and early osteoporosis screening in diabetic patients to reduce fracture risk and improve clinical outcomes.
--------------------------------------------------------------------------------
Introduction
Diabetes mellitus represents a major and escalating public health burden in India, with prevalence rates rising at an alarming pace. It is projected that the number of individuals affected by diabetes will increase from 40.6 million in 2006 to approximately 79.4 million by 2030 (1). This upward trend is likely to accelerate further as life expectancy increases and mortality from communicable diseases declines. Individuals with type 2 diabetes mellitus (T2DM) are predisposed to a broad spectrum of microvascular and macrovascular complications, the incidence and severity of which are largely determined by disease duration and the degree of glycemic control (2, 3, 4).
Beyond the well-recognized complications such as ischemic heart disease, cerebrovascular events, and diabetic nephropathy, mounting evidence indicates that individuals with T2DM are at a substantially increased risk of fractures compared to their non-diabetic counterparts. This elevated fracture risk has traditionally been attributed to diabetes-related complications, including hypoglycemia induced by antidiabetic therapy, impaired muscle strength, visual impairment secondary to diabetic retinopathy, peripheral arterial disease, and diabetic neuropathy, all of which contribute to imbalance and an increased propensity for falls. However, recent studies have demonstrated that diabetes exerts direct effects on bone metabolism at the molecular level, resulting in altered bone remodeling dynamics, increased bone resorption, and compromised bone quality (5, 6, 7).
Current evidence suggests a strong association between diabetes and reduced bone mineral density (BMD), manifesting as osteopenia and osteoporosis. This risk is further amplified in individuals with long-standing diabetes and those who develop chronic kidney disease (CKD), owing to disturbances in mineral metabolism, secondary hyperparathyroidism, and vitamin D deficiency (8, 9, 10). Despite the growing recognition of skeletal fragility in diabetes, data on the prevalence of osteopenia and osteoporosis among Indian patients with T2DM—particularly those with concomitant CKD—remain limited.
In view of these considerations, the present study was undertaken to estimate and compare the prevalence of osteopenia and osteoporosis in patients with T2DM with and without CKD and to contrast these findings with those observed in non-diabetic healthy controls.
Materials and Methods
Study Design and Setting
This hospital-based cross-sectional study was conducted between 2022 and 2023 through a collaborative effort between the Departments of Radiodiagnosis and Imaging and Medicine/Endocrinology at a tertiary care teaching hospital in the Kashmir Valley, India. Participants were recruited from the outpatient services of the Departments of Medicine and Endocrinology.
Participants
Eligible participants were adults aged ≥18 years attending the outpatient department who were approached consecutively and provided with detailed information regarding the study objectives and procedures. Written informed consent was obtained from all participants prior to enrollment. Patients diagnosed with T2DM, with or without diabetic kidney disease, were included. Healthy controls were selected from attendants of patients admitted for elective surgical procedures and were screened to ensure the absence of diabetes or other systemic illnesses affecting bone metabolism.
Case Definitions
Participants were categorized into three groups of 70 individuals each: T2DM with CKD, T2DM without CKD, and non-diabetic controls. The control group was age- and sex-matched and recruited from the same hospital setting to provide representative reference BMD values.
T2DM was defined by one or more of the following criteria: fasting plasma glucose ≥126 mg/dL, postprandial plasma glucose ≥200 mg/dL, glycated hemoglobin (HbA1c) ≥6.5%, or a prior diagnosis of diabetes with ongoing antidiabetic therapy.
Diabetic kidney disease was defined as urinary albumin excretion >15 µg, 24-hour urinary albuminuria >300 mg/day, or serum creatinine >120 µmol/L in a patient with diabetes.
Study Procedures
Following informed consent, all participants underwent a comprehensive baseline clinical evaluation and laboratory assessment. Sociodemographic and clinical data—including medical and surgical history, smoking and alcohol consumption, medication use, physical activity levels, and other factors influencing bone metabolism—were collected using a standardized questionnaire.
Laboratory investigations included fasting and postprandial plasma glucose, HbA1c, renal function tests, 24-hour urinary protein estimation, serum 25-hydroxyvitamin D, calcium, phosphorus, albumin, alkaline phosphatase, and thyroid-stimulating hormone levels. Bone mineral density of the lumbar spine and right femoral neck was assessed using dual-energy X-ray absorptiometry (DEXA).
Variables and Definitions
Bone mineral density was classified according to World Health Organization criteria: normal BMD (T-score ≥ −1), osteopenia (T-score between −1 and −2.5), and osteoporosis (T-score < −2.5). Body mass index (BMI) was calculated using standard formulas.
Sample Size
Sample size estimation was based on previous studies reporting the prevalence of osteoporosis in patients with T2DM. Assuming a power of 80% and a two-sided alpha of 0.05, a minimum sample size of 70 participants per group was calculated to detect statistically significant differences in BMD between patients with T2DM with and without CKD.
Statistical Analysis
Categorical variables were expressed as frequencies and percentages. The distribution of continuous variables was assessed using histograms, probability plots, and the Kolmogorov–Smirnov and Shapiro–Wilk tests. Normally distributed variables were summarized as mean ± standard deviation, while non-normally distributed variables were expressed as median with interquartile range. Comparisons between categorical variables were performed using Pearson’s χ² test or Fisher’s exact test, as appropriate. The Mann–Whitney U test was used for comparisons between two groups, and the Kruskal–Wallis test was applied for comparisons involving more than two groups. Statistical analyses were performed using Stata version 15.
Ethical Considerations
The study protocol was approved by the Institutional Ethics Committee. Written informed consent was obtained from all participants. Individuals younger than 18 years and those with cognitive impairment affecting decision-making capacity were excluded.
Results
Demographic and Clinical Characteristics
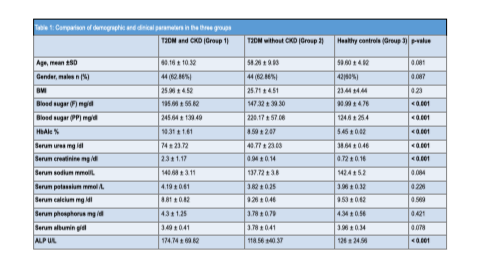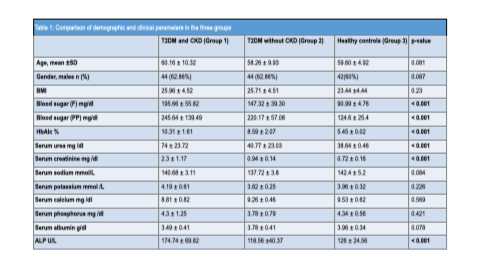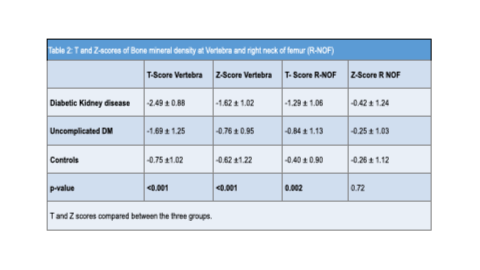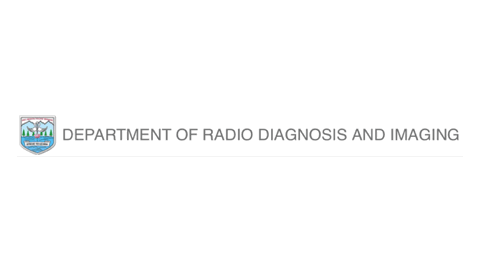
A total of 210 participants were included: 70 patients with T2DM and CKD (Group 1), 70 patients with T2DM without CKD (Group 2), and 70 healthy controls (Group 3). The three groups were comparable with respect to age and sex distribution. The mean age was 60.16 ± 10.32 years in Group 1, 58.26 ± 9.93 years in Group 2, and 59.60 ± 4.92 years in Group 3. Male participants constituted 62.86%, 62.86%, and 60% of Groups 1, 2, and 3, respectively. Body mass index did not differ significantly among the groups.
Serum electrolytes, calcium, phosphorus, and albumin levels were comparable across groups. However, fasting and postprandial glucose levels and HbA1c values were significantly higher in Group 1 compared to Group 2, with both groups exhibiting higher values than controls. Serum urea and creatinine levels were significantly elevated in patients with diabetic kidney disease.
Bone Mineral Density
Mean vertebral T-scores were −2.49 ± 0.88, −1.69 ± 1.25, and −0.75 ± 1.02 for Groups 1, 2, and 3, respectively, with corresponding Z-scores of −1.62 ± 1.02, −0.76 ± 0.95, and −0.62 ± 1.22. These differences were statistically significant.
At the right femoral neck, mean T-scores were −1.29 ± 1.06, −0.84 ± 1.13, and −0.40 ± 0.90 for Groups 1, 2, and 3, respectively, with corresponding Z-scores of −0.42 ± 1.24, −0.25 ± 1.03, and −0.26 ± 1.12. The differences in T-scores were statistically significant.
Discussion
Diabetes mellitus and osteoporosis are prevalent chronic disorders that substantially impair quality of life, particularly among older adults (11). Diabetes and diabetic kidney disease induce complex metabolic and hormonal alterations that adversely affect bone turnover, leading to reduced bone mineral density and increased skeletal fragility (12, 13). When combined with an elevated risk of falls due to neuropathy and visual impairment, these changes significantly increase fracture risk in diabetic individuals (14, 15).
The present study demonstrated a markedly higher prevalence of osteopenia and osteoporosis among patients with T2DM, particularly those with concomitant CKD, compared to non-diabetic controls. Vertebral osteoporosis and osteopenia were observed in 54% and 38% of patients with diabetic kidney disease, respectively, whereas patients with T2DM without CKD exhibited lower prevalence rates. The control group showed substantially lower rates of both conditions. These findings are consistent with prior studies that have reported an increased burden of osteoporosis in patients with diabetes, especially in the presence of CKD (16, 17, 18, 19, 20).
At the femoral neck, osteoporosis prevalence was highest among patients with diabetic kidney disease, followed by those with uncomplicated diabetes and controls. This is clinically significant, as femoral neck fractures are associated with considerable morbidity, loss of independence, and increased mortality. Our findings corroborate previous reports demonstrating reduced femoral neck BMD in patients with diabetic kidney disease (21, 22, 23, 24, 25). Collectively, these results underscore the need for early identification and proactive management of bone disease in patients with diabetes. Routine screening for osteoporosis in patients with long-standing diabetes, particularly those with CKD, may facilitate timely intervention, reduce fracture risk, and improve long-term functional outcomes.
Conclusion
This study demonstrates a significantly higher prevalence of osteopenia and osteoporosis among patients with T2DM, with the greatest burden observed in those with concomitant CKD. Both vertebral and femoral neck bone mineral density were significantly lower in diabetic patients compared to non-diabetic controls. These findings highlight the importance of routine osteoporosis screening and early bone health assessment in diabetic patients, particularly those with diabetic kidney disease, to mitigate fracture risk and prevent associated functional impairment.
References
1. Ranasinghe P, Jayawardena R, Gamage N, Sivanandam N, Misra A. Prevalence and trends of the diabetes epidemic in urban and rural India: A pooled systematic review and meta-analysis of 1.7 million adults. Annals of epidemiology. 2021 Jun 1;58:128-48. https://doi.org/10.1016/j.annepidem.2021.02.016
2. Luhar S, Timæus IM, Jones R, Cunningham S, Patel SA, Kinra S, Clarke L, Houben R. Forecasting the prevalence of overweight and obesity in India to 2040. PloS one. 2020 Feb 24;15(2):e0229438. https://doi.org/10.1371/journal.pone.0229438
3. Kumar AS, Sinha N. Cardiovascular disease in India: a 360 degree overview. Medical Journal Armed Forces India. 2020 Jan 1;76(1):1-3. https://doi.org/10.1016/j.mjafi.2019.12.005
4. Paul S, Ali A, Katare R. Molecular complexities underlying the vascular complications of diabetes mellitus–A comprehensive review. Journal of Diabetes and its Complications. 2020 Aug 1;34(8):107613. https://doi.org/10.1016/j.jdiacomp.2020.107613
5. Balaji R, Duraisamy R, Kumar MP. Complications of diabetes mellitus: A review. Drug Invention Today. 2019 Jan 15;12(1). https://www.researchgate.net/publication/332569443_
6. Bai J, Gao Q, Wang C, Dai J. Diabetes mellitus and risk of low-energy fracture: a meta-analysis. Aging clinical and experimental research. 2020 Nov;32:2173-86. https://doi.org/10.1007/s40520-019-01417-x
7. Eller-Vainicher C, Cairoli E, Grassi G, Grassi F, Catalano A, Merlotti D, Falchetti A, Gaudio A, Chiodini I, Gennari L. Pathophysiology and management of type 2 diabetes mellitus bone fragility. Journal of Diabetes Research. 2020 May 23;2020. https://doi.org/10.1155/2020/7608964
8. Ala M, Jafari RM, Dehpour AR. Diabetes mellitus and osteoporosis correlation: challenges and hopes. Current Diabetes Reviews. 2020 Nov 1;16(9):984-1001. https://doi.org/10.2174/1573399816666200324152517
9. Kovesdy CP. Epidemiology of chronic kidney disease: an update 2022. Kidney International Supplements. 2022 Apr 1;12(1):7-11. https://doi.org/10.1016/j.kisu.2021.11.003
10. Liu J, Ren ZH, Qiang H, Wu J, Shen M, Zhang L, Lyu J. Trends in the incidence of diabetes mellitus: results from the Global Burden of Disease Study 2017 and implications for diabetes mellitus prevention. BMC public health. 2020 Dec;20:1-2. https://doi.org/10.1186/s12889-020-09502-x
11. Cheng HT, Xu X, Lim PS, Hung KY. Worldwide epidemiology of diabetes-related end-stage renal disease, 2000–2015. Diabetes Care. 2021 Jan 1;44(1):89-97. https://doi.org/10.2337/dc20-1913
12. Shetty S, John B, Mohan S, Paul TV. Vertebral fracture assessment by dual-energy X-ray absorptiometry along with bone mineral density in the evaluation of postmenopausal osteoporosis. Archives of Osteoporosis. 2020 Dec;15:1-6. https://doi.org/10.1007/s11657-020-0688-9
13. Messina C, Albano D, Gitto S, Tofanelli L, Bazzocchi A, Ulivieri FM, Guglielmi G, Sconfienza LM. Body composition with dual energy X-ray absorptiometry: from basics to new tools. Quantitative imaging in medicine and surgery. 2020 Aug;10(8):1687. https://doi.org/10.21037/qims.2020.03.02
14. Walker MD, Williams J, Lewis SK, Bai JC, Lebwohl B, Green PH. Measurement of forearm bone density by dual energy x-ray absorptiometry increases the prevalence of osteoporosis in men with celiac disease. Clinical Gastroenterology and Hepatology. 2020 Jan 1;18(1):99-106. https://doi.org/10.1016/j.cgh.2019.03.049
15. Freiman JA, Chalmers TC, Smith HA, Kuebler RR. The importance of beta, the type II error, and sample size in the design and interpretation of the randomized controlled trial: survey of two sets of “negative” trials. InMedical uses of statistics 2019 May 20 (pp. 357-389). CRC Press. https://www.taylorfrancis.com/chapters/edit/10.1201/9780429187445-19/
16. Johnell O, Kanis JA. An estimate of the worldwide prevalence and disability associated with osteoporotic fractures. Osteoporosis international. 2006 Dec;17:1726-33. https://doi.org/10.1007/s00198-006-0172-4
17. Adil C, Aydın T, Taşpınar Ö, Kızıltan H, Eriş AH, Hocaoglu IT, Poşul S, Kepekci M, Denizli E, Güler M. Bone mineral density evaluation of patients with type 2 diabetes mellitus. Journal of physical therapy science. 2015;27(1):179-82. https://doi.org/10.1589/jpts.27.179
18. Chen H, Li J, Wang Q. Associations between bone-alkaline phosphatase and bone mineral density in adults with and without diabetes. Medicine. 2018 Apr;97(17). https://doi.org/10.1097/MD.0000000000010432
19. Asokan AG, Jaganathan J, Philip R, Soman RR, Sebastian ST, Pullishery F. Evaluation of bone mineral density among type 2 diabetes mellitus patients in South Karnataka. Journal of natural science, biology, and medicine. 2017 Jan;8(1):94. https://doi.org/10.4103/0976-9668.198363
20. Jang M, Kim H, Lea S, Oh S, Kim JS, Oh B. Effect of duration of diabetes on bone mineral density: a population study on East Asian males. BMC endocrine disorders. 2018 Dec;18:1-9. https://doi.org/10.1186/s12902-018-0290-y
21. Huang JF, Zheng XQ, Sun XL, Zhou X, Liu J, Li YM, Wang XY, Zhang XL, Wu AM. Association between bone mineral density and severity of chronic kidney disease. International journal of endocrinology. 2020 Oct 28;2020. https://doi.org/10.1155/2020/8852690
22. Yenchek RH, Ix JH, Shlipak MG, Bauer DC, Rianon NJ, Kritchevsky SB, Harris TB, Newman AB, Cauley JA, Fried LF, Health, Aging, and Body Composition Study. Bone mineral density and fracture risk in older individuals with CKD. Clinical Journal of the American Society of Nephrology. 2012 Jul 1;7(7):1130-6. https://doi.org/10.2215/CJN.12871211
23. Gad SA, Elagrody AI. Are Diabetes Mellitus and Diabetic Nephropathy Good Predictors of Osteoporosis. The Egyptian Journal of Hospital Medicine. 2021 Jan 1;82(3):497-501.
24. Malmgren L, McGuigan F, Christensson A, Akesson KE. Reduced kidney function is associated with BMD, bone loss and markers of mineral homeostasis in older women: a 10-year longitudinal study. Osteoporosis International. 2017 Dec;28:3463-73. https://doi.org/10.1007/s00198-017-4221-y
25. Miller PD. Bone disease in CKD: a focus on osteoporosis diagnosis and management. American journal of kidney diseases. 2014 Aug 1;64(2):290-304. https://doi.org/10.1053/j.ajkd.2013.12.018
Downloads
Published
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request. The data contain potentially identifiable patient information and are therefore not publicaly available in accordance with Institutional review board and data protection regulations.
License
Copyright (c) 2026 Surjeet Chandail, Arshed Hussain Parry, Majid Jehangir, Syeed Aalishan Fatima, Seema Qayoom

This work is licensed under a Creative Commons Attribution 4.0 International License.
This license requires that reusers give credit to the creator. It allows reusers to distribute, remix, adapt, and build upon the material in any medium or format, even for commercial purposes.








