From Fibroscan to 2D Shear Wave Elastography
Technological Evolution or Diagnostic Substitution in Liver Fibrosis Assessment?
DOI:
https://doi.org/10.59667/sjoranm.v26i1.16Keywords:
Liver fibrosis, Fibroscan, Shear wave elastography, CLDAbstract
AbstractBackground: Proper assessment of liver fibrosis is essential for treating chronic liver disease (CLD). While Fibroscan is commonly used, it often fails in patients with obesity or ascites. Two-dimensional shear wave elastography (2D SWE) adds real-time ultrasound imaging, which may improve its success and reliability in these challenging cases.
Objective: We aimed to compare how well Fibroscan and 2D SWE detect and stage liver fibrosis in CLD patients, using liver biopsy results as the gold standard.
Methods: In this prospective, hospital based study at Government Medical College Srinagar, we enrolled 155 CLD patients undergoing liver biopsies from October 2022 to November 2025. Independent blinded operators performed Fibroscan with M-probe and 2D SWE on each patient. Liver stiffness was measured in kilopascals (kPa) and compared it to METAVIR fibrosis scores . Dignostic accuracy, agreement rates, and technical failure rates were calculated.
Results: Out of 155 enrolled patients, 136 patients completed all evaluations (Fibroscan , SWE & Liver biopsy). Fibroscan failed in 18 of 155 patients (11.6%) mainly due to obesity or ascites, while 2D SWE failed in 8 of 155 patients (5.16%). Notably, SWE achieved valid results in 11 out of 18 patients (61%) in whom fibroscan failed. The two methods agreed 86.8% of the time supported by weighted kappa of 0.893(95% CI : 0.82-0.92) indicating almost perfect agreement as per Landis-Koch criteria : k>0.81 and confirmed by Pearson’s chi-square test ( X2 = 371.22 , p <0.0001). Diagnostic performance metrics revealed high accuracy for both modalities across fibrosis stage F0-F4 with Efficacy exceeding 93% for all stages, though SWE tended to slightly overcall F2–F3 stages yet overall performance was similar. Procedure times were alike (Fibroscan: 5.3 ± 2.2 min; SWE: 5.4 ± 2.5 min).
Conclusion: 2D SWE matches Fibroscan’s accuracy but with fewer failures and advantage of real time anatomical guidance, making it a viable alternative especially for obese or ascitic CLD patients.
---------------------------------------------
Introduction
Chronic liver disease is a substantial worldwide problem. Its major consequence is increasing deposition of fibrous tissue within the liver, leading to the development of cirrhosis with its consequences, portal hypertension, hepatic insufficiency, and hepatocellular carcinoma (HCC). Different histologic stages of progressive liver fibrosis have been described, from no fibrosis (METAVIR stage F0) to the cirrhotic stage (METAVIR stage F4). As fibrosis progresses, there is increasing portal hypertension, loss of liver function, and higher risk of HCC. The stage of liver fibrosis is important to determine prognosis and surveillance and to prioritize for treatment and potential for reversibility. The process of fibrosis is dynamic, and studies have shown that a regression of fibrosis is possible with treatment of the underlying condition (eg, antiviral therapy in viral hepatitis and immunosuppression in autoimmune hepatitis) [1-3]. Liver biopsy remains the gold standard for assessing the degree of hepatic fibrosis. However it is fraught with certain limitations , including its invasive nature , associated procedure-related complications and high cost. [4, 5]. Further, tissue obtained via biopsy represents roughly only 1/50000 of the liver volume, which may result in sampling error [6]. Consequently, there has been a growing interest to develop alternate non-invasive tools that can accurately predict hepatic fibrosis without the need for biopsy.
Various non-invasive methods for evaluating liver fibrosis have rapidly improved as alternatives to liver biopsy with fibroscan the commonest non-invasive tool used as an alternative for biopsy. It is a non-invasive, bedside, and rapid test [7]. Fibroscan probe uses a mechanical vibration that creates shear wave within the liver parenchyma and also ready to read the velocity reflected from the liver surface which shows changes according to the degree of liver fibrosis, then giving a measurement reflecting the degree of liver stiffness which is displayed in kilopascal (kPa) [8]. However, technical failure occurs in patients with obesity, ascites, or anatomical obstacles [9].
2D SWE is relatively recent tool in the era of liver fibrosis grading as a non-invasive tool which uses the usual 2D US probe, yet with production of a focused acoustic beam to generate shear wave within the liver with the degree of liver fibrosis reflected upon the speed of this wave within the liver parenchyma and affects the degree of reflected wave. The same probe can track the reflected wave then calculate the degree of liver fibrosis using the machine software which is presented in kPa after conversion [10, 11].
This study analyses whether 2D SWE represents an evolutionary improvement or a true substitution for Fibroscan in liver stiffness assessment in CLD.
Materials and Methods
Study Population
This prospective study was done in the department of Radiodiagnosis & Imaging in collaboration with department of Gastroenterology and department of Pathology, Government Medical College Srinagar, India over a period of 3 years between October 2022 and November 2025 and included 155 patients with chronic liver disease, scheduled for liver fibrosis staging.
Exclusion criteria included heart failure, acute hepatitis, hepatic vascular thrombosis, focal lesions, prior interventions, or incomplete biopsy data. Ethical approval and informed consent were obtained.
Elastography Techniques
Fibroscan/Transient Elastography using EchosenseFibroscan Expert machine:
Examinations were performed with patients in the supine position, right arm maximally abducted. The M or XL probe was selected according to body habitus. Ten valid measurements were obtained through the right midaxillary line, avoiding large vessels. Validity criteria: interquartile range (IQR)/median <30%. Median stiffness values expressed in kilopascals (kPa) were recorded.
2D Shear Wave Elastography Using EsaoteMyLab™ X90 machine:
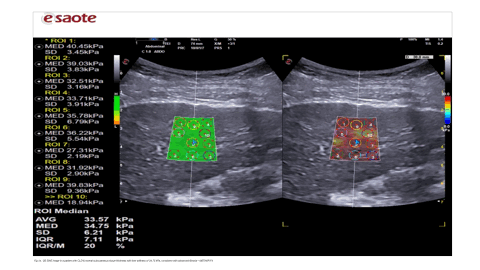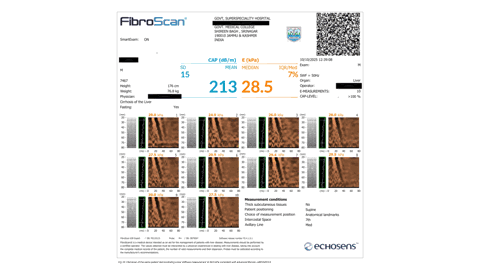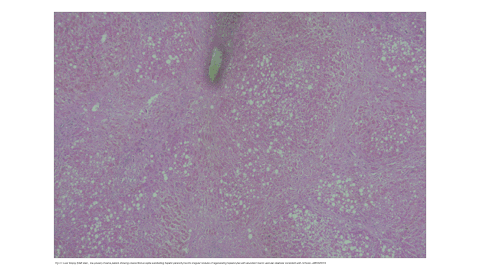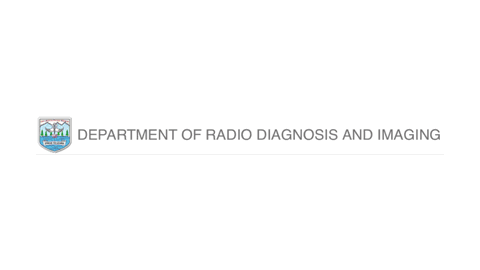
2D SWE was performed using a convex probe (1–6 MHz) with B-mode ultrasound guidance. Measurements were obtained from the right liver lobe, at least 2 cm below the capsule, avoiding vascular structures. 9–12 valid measurements were recorded, with IQR/median <30% for reliability. Stiffness values were automatically converted to kPa using inbuilt software.
Operator blinding: Each elastography method was performed by a separate experienced operator blinded to both the biopsy and the other elastography results.
Histological evaluation
All liver biopsy specimens were obtained percutaneously from the right lobe of liver using 18-G needles with core length of 2 cms under ultrasound guidance by two radiologists with sufficient knowledge and experience in percutaneous USG guided biopsies.
Specimens were fixed, embedded in paraffin, and stained with hematoxylin–eosin, Azan and Gitter stains. Two pathologists with sufficient knowledge and experience in hepatic pathology who were blinded to imaging data evaluated the liver biopsy samples. If there was a discrepancy, they discussed the results and reached a consensus. Fibrosis was staged according to the METAVIR scoring system.
F0: No fibrosis.
F1: Portal fibrosis without septa.
F2: Portal fibrosis with few septa.
F3: Numerous septa without cirrhosis.
F4: cirrhosis.
Statistical Analysis
The analysis of data was done using SPSS version 24.0 with results rounded to one decimal. Categorical variables were expressed as frequencies and percentages, and numeric variables as mean ± SD. Inter-method agreement (2D SWE vs Fibroscan) was evaluated via weighted kappa statistic (Table 3). The guidelines of Landis and Koch were followed to interpret k values: 0.00-0.20 indicated slight agreement; 0.21-0.40, fair agreement; 0.41-0.60, moderate agreement; 0.61-0.80, substantial agreement, 0.81-1.00, almost perfect agreement. Pearson’s chi-square test of independence was used to assess the association between fibroscan SWE fibrosis staging classifications using fibroscan results as reference (Table 3). Statistical significance was defined as p <0.05 , values p < 0.001 were considered highly significant . Sensitivity, specificity, positive, negative predictive values and efficacy of SWE and fibroscan were calculated for each stage of Liver fibrosis in comparison to Liver biopsy (Table 4)
Results
Out of 155 enrolled patients, 136 completed the study to the analysis level. Fibroscan failed in 18 of 155 patients (11.6%), primarily due to obesity or ascites. Of the Fibroscan failures, 11 patients (61%) were successfully assessed using 2D SWE. 2D SWE failed in 8 of 155 patients (5.16%), mainly from steatosis or motion artefacts.
The mean age of the selected patients was 52 ± 7 years SD. Eighty four patients out of 136 were male representing 61.76% of the cases. Fifty two patients out of 136 were female representing 38.24 % of the cases.
Fibroscan and 2D SWE both showed very good diagnostic performance across the METAVIR spectrum, with close concordance to histological staging and to each other.
Fibroscan demonstrated exact stage agreement with liver biopsy in 123/136 cases(90.4%) while SWE achieved agreement in 120/136 cases(88.2%) (Table 1&2).
Intermethod Concordance between 2D SWE and fibroscan was observed in 118/136 cases (86.8%), supported by weighted kappa of 0.893(95% CI: 0.82-0.92) indicating almost perfect agreement as per Landis-Koch criteria: k>0.81 and confirmed by Pearson’s chi-square test (X2 = 371.22, p <0.0001) (Table 3)
Diagnostic performance metrics revealed high accuracy for both modalities across fibrosis stage F0-F4. Efficacy exceeded 93% for all stages (Fibroscan: 94.9-97.8%, SWE: 93.4- 97.1%). SWE showed slightly higher sensitivity in F0 and F4, whereas Fibroscan demonstrated superior sensitivity in F1 and F3. For F2, the sensitivities of both modalities were comparable. Specificity remained consistently high for both tests (>95%) across all stages. PPV varied by stage, with Fibroscan outperforming SWE in most stages except F3, where SWE showed higher PPV. Negative predictive values were uniformly high for both modalities (>95%). Overall accuracy ranged from 93% to 98% for both methods, with no major performance differences observed between Fibroscan and 2D SWE (Table 4).
Fibroscan showed minimal understaging and overstaging relative to liver biopsy with most discrepancies limited to one fibrosis grade. Fibroscan understaged fibrosis in 4 patients (2.9% cases) and overstaged fibrosis in in 9 patients (6.6% cases). Fibroscan under-staging occurred mainly in intermediate stages (F2–F3), while over-staging was generally limited to single-stage shifts.
2D SWE showed a comparable pattern of discrepancies, understaging fibrosis in 3 patients (2.2% cases) and overstaging fibrosis in 11 patients (8.0% cases). SWE showed under-staging primarily in early fibrosis (F1–F2) and over-staging mainly around the F2–F3 transition.
SWE showed a higher incidence of overstaging and lower incidence of understaging.
Exam duration was comparable (Fibroscan: 5.3 ± 2.2 min; SWE: 5.4 ± 2.5min) with no significant difference between the two techniques regarding the duration of the technique.
Discussion
Chronic Liver disease represents a major global health burden, characterized by progressive fibrous tissue deposition leading to cirrhosis , portal hypertension , hepatic decompensation and hepatocellular carcinoma (HCC). Accurate fibrosis staging is crucial for prognosis, treatment, prioritizing and monitoring reversibility with etiology- specific therapies such as antivirals or immunosuppression. Liver biopsy remains the gold standard for the evaluation of the liver fibrosis, yet its invasive nature, high cost, sampling errors due to limited tissue representations necessitate reliable non-invasive alternatives like fibroscan and 2D SWE.
This prospective study at Government Medical College Srinagar compared Fibroscan and 2D SWE in 155 patients with chronic liver disease. Both modalities showed strong concordance with biopsy findings. Fibroscan is considered one of the most used alternatives for biopsy and already put in patient’s management algorithms in most of the European countries. However, some limitation was observed with the use of fibroscan regarding obesity and ascites (8, 12).
SWE is a relatively new technique that can be utilised as part of the standard ultrasound examination to assess the presence of liver fibrosis or cirrhosis [13]. The use of the SWE method offers the advantage of being integrated into a conventional ultrasound system. It can be performed during a standard B-mode liver ultrasound, which is commonly used for the follow-ups of patients suffering from a chronic liver disease. In addition, the advantages offered by the SWE include the ability to choose the positioning of the ROI in the liver, thus avoiding interfering structures such as large blood vessels and bile ducts. In addition, the SWE method is not influenced by obesity or ascites.
In our study Fibroscan failed in 18 of 155 patients (11.6%) mainly due to obesity or ascites, while 2D SWE failed in 8 of 155 patients (5.16%). SWE achieved valid results in 11 out of 18 patients (61%) in whom fibroscan failed. This is already consistent with similar studies as Osman, Ahmed M et al [14]. who reported a failure rate of fibroscan reaching 14.3% and Castera et al [15] who reported a failure rate of fibroscan reaching 20% with overall 5 years experience with patient’s obesity considered one of the most important causes of technique failure. Osman, Ahmed M et al [14] recorded less failure rate of SWE compared with fibroscan with SWE failure rate 6.7% which is consistent with our results. The lower failure rate of SWE can be explained by the simultaneous B-mode visualization available during the SWE technique which provides the opportunity to select the proper site for reading away from the ascites interface and can avoid areas of obesity which was more common in the axillary region rather than the anterior chest wall in our patients. This is in controversy to the fibroscan which depends on the application of the probe blindly on a specific anatomical level on the patient’s body at the midaxillary level which showed more fat level rather than the anterior chest wall [9, 15, 16].
In our study Fibroscan and SWE both showed very good diagnostic performance across the METAVIR spectrum, with close concordance to histological staging and to each other but SWE showed a tendency to slightly overestimate the fibrosis stage relative to fibroscan (8.0% in SWE Vs 6.6% in fibroscan) with overstaging mainly around the F2-F3 transition, SWE however showed lower incidence of understaging relative to fibroscan. These results are consistent with previous research by Osman, Ahmed M et al[14] ,O’Hara et al [17] , Foucher J et al [18] and Zeng J et al [19] suggesting SWE's sensitivity to focal stiffness heterogeneity.
Exam times were similar, suggesting that SWE’s advantages in anatomical guidance do not reduce efficiency. SWE’s integration into routine ultrasound platforms also supports cost-effective, comprehensive evaluation.
This study confirms 2D SWE as a viable substitute for Fibroscan in liver fibrosis staging with equivalent diagnostic accuracy and improved technical success, particularly in obese (Fig 2a , 2b & 2c) or ascitic (Fig 3a , 3b & 3c) patients.
Guidelines from European Federation of Societies for Ultrasound in Medicine and Biology (EFSUMB) , World Federation for Ultrasound in Medicine and Biology (WFUMB), and European Association for the Study of the Liver (EASL) support both modalities with choice of modality determined by patient habitus and clinical context [13, 20]. Operator training and device-specific cut-offs remain critical to optimize reliability.
Limitations in this study include single-center design, lack of interobserver reproducibility analysis, and minor sample attrition due to technical failure. Nevertheless, the results support the routine use of 2D SWE in most CLD scenarios, particularly where Fibroscan is inappropriate or unsuccessful.
Conclusion
2D SWE and Fibroscan provide comparable liver stiffness assessment in patients with chronic liver disease. 2D SWE offers practical edge in patients with obesity/ascites, and facilitates a broader, more anatomically precise assessment. SWE can be considered not merely an evolution, but a viable alternative for Fibroscan as the non-invasive standard for liver fibrosis staging in CLD especially in obese/ascitic patients.
References
1. Marcellin P, Gane E, Buti M, et al. Regression of cirrhosis during treatment with tenofovir disoproxil fumarate for chronic hepatitis B: a 5-year open-label follow-up study. Lancet 2013;381(9865):468–475. https://doi.org/10.1016/S0140-6736(12)61425-1
2. Carrión JA, Navasa M, García-Retortillo M, et al. Efficacy of antiviral therapy on hepatitis C recurrence after liver transplantation: a randomized controlled study. Gastroenterology 2007;132(5):1746–1756. https://doi.org/10.1053/j.gastro.2007.03.041
3. Martinez SM, Foucher J, Combis JM, et al. Longitudinal liver stiffness assessment in patients with chronic hepatitis C undergoing antiviral therapy. PLoS ONE 2012;7(10):e47715. https://doi.org/10.1371/journal.pone.0047715
4. Seeff LB, Everson GT, Morgan TR, et al. Complication rate of percutaneous liver biopsies among persons with advanced chronic liver disease in the HALT-C trial. Clin Gastroenterol Hepatol 2010;8(10):877–883. https://doi.org/10.1016/j.cgh.2010.03.025
5. Stotland BR, Lichtenstein GR. Liver biopsy complications and routine ultrasound. Am J Gastroenterol 1996;91(7):1295–1296. https://pubmed.ncbi.nlm.nih.gov/8677980/
6. Regev A, Berho M, Jeffers LJ, et al. Sampling error and intraobserver variation in liver biopsy in patients with chronic HCV infection. Am J Gastroenterol 2002;97 (10):2614–2618. https://doi.org/10.1111/j.1572-0241.2002.06038.x
7. Ali Z, Zytoon A, Elsakhawy M, et al. Real-time shear wave elastography for assessing liver fibrosis in patients with chronic hepatitis C. Menoufia Medical Journal. 2018;31(5):385-393.
8. Shiina T, Nightingale KR, Palmeri ML, Hall TJ, Bamber JC, Barr RG et al. WFUMB guidelines and recommendations for clinical use of ultrasound elastography: Part 1: Basic principles and terminology. Ultrasound Med Biol 2015; 41: 1126–1147. https://doi.org/10.1016/j.ultrasmedbio.2015.03.009
9. Guibal A, Renosi G, Rode A, Scoazec JY, Guillaud O, Chardon L et al. Shear wave elastography: An accurate technique to stage liver fibrosis in chronic liver diseases. Diagn Interv Imaging 2016; 97: 91–99. https://doi.org/10.1016/j.diii.2015.11.001
10. Tada T, Kumada T, Toyoda H, et al. Utility of real-time shear wave elastography for assessing liver fibrosis in patients with chronic hepatitis C infection without cirrhosis: comparison of liver fibrosis indices. Hepatol Res. 2015;45(10):1221-1229. doi:10.1111/hepr.12490 https://doi.org/10.1111/hepr.12476
11. Song P, Mellema DC, Sheedy SP, Meixner DD, Karshen RM, Urban MW, Manduca A, Sanchez W, Callstrom MR, Greenleaf JF, Chen S. Performance of 2-dimensional ultrasound shear wave elastography in liver fibrosis detection using magnetic resonance elastography as the reference standard: a pilot study. J Ultrasound Med. 2016;35(2):401–412. https://doi.org/10.7863/ultra.15.03036
12. European Association for the Study of the Liver (EASL). EASL clinical practice guidelines: management of hepatitis C virus infection. J Hepatol. 2011;55(2):245-264. https://doi.org/10.1016/j.jhep.2011.02.023
13. Barr RG, Ferraioli G, Palmeri ML, Goodman ZD, Garcia-Tsao G, Rubin J et al. Elastography assessment of liver fibrosis: Society of radiologists in ultrasound consensus conference statement. Radiology 2015; 276: 845–861. https://doi.org/10.1148/radiol.2015150619
14. Osman, Ahmed M et al. “2D shear wave elastography (SWE) performance versus vibration-controlled transient elastography (VCTE/ fibroscan) in the assessment of liver stiffness in chronic hepatitis.”Insights into imaging vol. 11,1 38. 10 Mar. 2020, https://doi.org/10.1186/s13244-020-0839-y
15. Castera L, Foucher J, Bernard P, et al. Pitfalls of liver stiffness measurement: a 5-year prospective study of 13,369 examinations. Hepatology. 2010;51(3):828-835. https://doi.org/10.1002/hep.23425
16. Pavlov CS, Casazza G, Nikolova D, et al. Transient elastography for diagnosis of stages of hepatic fibrosis and cirrhosis in people with alcoholic liver disease. Cochrane Database Syst Rev. 2015;1: CD010542. https://doi.org/10.1002/14651858.CD010542.pub2
17. O'Hara Sandra, Hodson Susan, Hernaman Chandelle, Wambeek Nick, Olynyk John. Concordance of transient elastography and shear wave elastography for measurement of liver stiffness. Sonography. 2017;4(4):141–145. https://doi.org/10.1002/sono.12122
18. Foucher J, Chanteloup E, Vergniol J, et al. Diagnosis of cirrhosis by transient elastography (FibroScan): a prospective study. Gut. 2006;55(3):403-408. https://doi.org/10.1136/gut.2005.069153
19. Zeng J, Zheng J, Huang Z, et al. Comparison of 2D shear wave elastography and transient elastography for assessment of liver fibrosis in chronic hepatitis B. Ultrasound Med Biol. 2017;43(8):1563-1570. https://doi.org/10.1016/j.ultrasmedbio.2017.03.014
20. Ryu H, Ahn SJ, Yoon JH, Lee JM. Reproducibility of liver stiffness measurements made with two different 2-dimensional shear wave elastography systems using the comb-push technique. Ultrasonography. 2019;38(3):246-254. https://doi.org/10.14366/usg.18046
Downloads
Published
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request. The data contain potentially identifiable patient information and are therefore not publicaly available in accordance with Institutional review board and data protection regulations.
License
Copyright (c) 2025 Dr Akib Arfee, Dr. Omair Ashraf Shah, Dr Asif Iqball , Dr Afshana Bashir, Dr Aaqib Manzoor

This work is licensed under a Creative Commons Attribution 4.0 International License.
This license requires that reusers give credit to the creator. It allows reusers to distribute, remix, adapt, and build upon the material in any medium or format, even for commercial purposes.








